The Technology of Photoresist Stripping
Stripping of most photoresist is, essentially, just an acid/base neutralization process. Yet photoresist stripping is usually unnecessarily costly, misunderstood, often a process bottleneck, and a source of many reject boards, and that’s on the good days. We will attempt to analyze why these things occur at the molecular level, and offer some cures.
Stripping problems usually are the result of two performance deficiencies, either failure of the anti-tarnish system and/or wide variance of stripping speed and quality, with use of the stripper.
Most photoresist strippers start off giving adequate anti-tarnish performance when the stripper is fresh. At some time during the life of the stripper the panels start coming out tarnished. This is frequently thought to be the result of the anti-tarnish chemistry being consumed. This is not the case. The real reason that this occurs is that dissolved copper in the stripper is a catalyst which accelerates the tarnishing of the copper metal substrate. As the stripper is used, the dissolved copper content builds, and the stripper becomes increasingly corrosive to the exposed copper metal substrate.
Thus the tarnishing of panels in a used stripper is, in reality, a failure of the anti-tarnish chemistry to deal with the increased corrosivity of the copper contaminated stripper, and can be avoided by choosing a stripper with anti-tarnish chemistry robust enough to overcome the corrosivity of very high levels of dissolved copper. This choice can result in saving large amounts of money on stripper chemistry, as the stripper can then be used to its real capacity rather than being prematurely dumped because it is tarnishing the copper. This is the more obvious problem.
The dramatic decrease in speed of stripping with usage of the stripping chemistry, is another source of truly excessive stripping chemistry cost, and worse, of many rejects in the manufacturing process. It is usually the result of using general purpose resist strippers, and/or inappropriate control methods.
The general chemistry of most resist strippers used by the PCB manufacturers in the US is very similar, even though it comes in drums with different labels. Further many of the PCB manufactures are using the same stripping chemistry for stripping inner and outerlayers, and most of them using this type of chemistry are wasting 50% of the money that they are spending on resist stripping, and getting unpredictable stripping in the bargain. This is not a pretty picture.
Admittedly, these are some pretty brassy claims, but they are unfortunately too true, and I will proceed to show you why this occurs.
Photoresist is acidic, and the stripping process neutralizes thisacidity, and in the process of neutralization the alkalinity of the resist stripper is consumed. The neutralized fragments are either dissolved or dispersed into the stripper solution. What these neutralizing agents are, and how they work determines the quality and speed of stripping.
The two most popular resist strippers in the US have essentially the same stripping chemistry. This chemistry is a compromise chemistry that does a good job on outerlayers for the first 20%, or so of it’s life, and then is only really adequate for stripping innerlayers over the rest of it’s life.
Specifically the principal stripping ingredients in these two popular strippers are Choline and Monoethanolamine (MEA). Choline is one of a class of organics called “phase transfer catalysts”, and it can be described as an organic caustic. In the normal inorganic caustics, the active part of the molecule is the hydroxide [ ], while the Sodium or Potassium is merely along to neutralize the charge on the [
], while the Sodium or Potassium is merely along to neutralize the charge on the [ ]. Choline contains the same hydroxide [
]. Choline contains the same hydroxide [ ], but the inorganic Sodium or Potassium is replaced by an organic amine with a positive charge.
], but the inorganic Sodium or Potassium is replaced by an organic amine with a positive charge.

Figure 1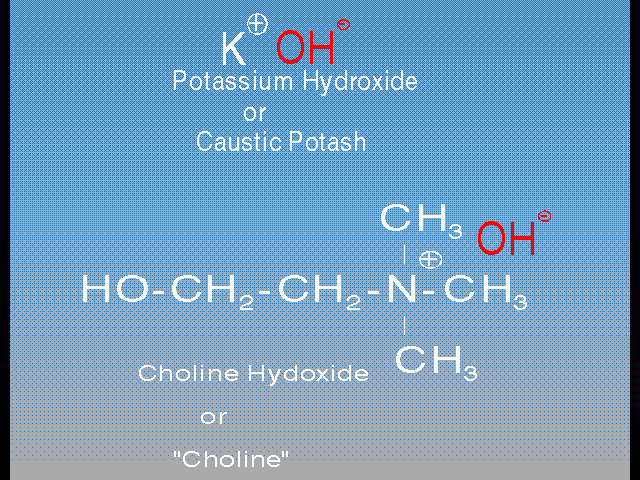
This “organic caustic” is called a phase transfer catalyst because it can carry (transfer) the caustic end of the molecule, the hydroxide [ ], from the water “phase” to an organic “phase”, like photoresist. In other words this little gem can actually facilitate the caustic dissolving in the resist itself. This is why Choline is the best thing that has ever happened to stripping photoresist. The Choline actually carries the caustic into the film, thus destroying the chemical bonds within the film, which breaks the film up quickly.
], from the water “phase” to an organic “phase”, like photoresist. In other words this little gem can actually facilitate the caustic dissolving in the resist itself. This is why Choline is the best thing that has ever happened to stripping photoresist. The Choline actually carries the caustic into the film, thus destroying the chemical bonds within the film, which breaks the film up quickly.
There is however one tiny problem, Choline is very, very expensive. It costs something like 10 times the cost of MEA per square foot of resist stripped. Again, Choline costs something like 10 times the cost of MEA per square foot of resist stripped. Monoethanolamine (MEA) reacts with the water in solution to produce the hydroxide [ ] by this reaction:
] by this reaction:

Figure 2
Now that we have covered the technical stuff, here is the critical fact that explains why strippers slow so dramatically so early in their life. Choline and MEA are very different strength bases, and because of this they are consumed sequentially (one after the other) in the stripping process, rather than together. In other words, all the Choline is completely consumed before any MEA is consumed. This results in a stripper of widely varying performance, as the stripper runs out of Choline, and shifts to stripping completely with MEA, the quality of strippiong decreases dramatically.
As we have all experienced, these widely varying performance characteristics can result in widely varying results, which are also known, in more polite circles, as rejects.
It is now time to get real real. The formula of the two largest selling resist strippers is approximately the following:

Figure 3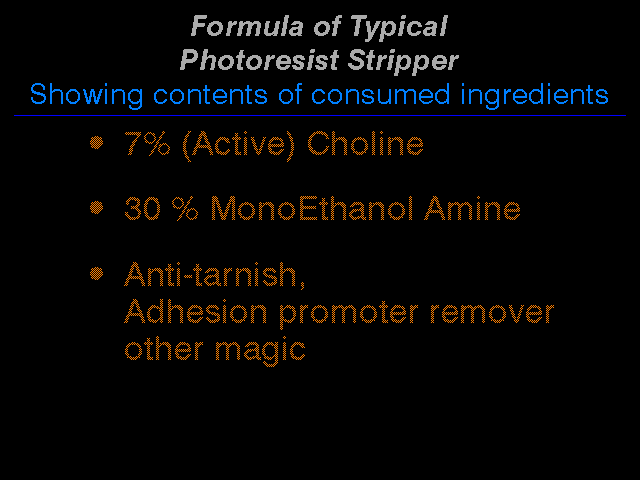
The interesting thing is that the 7% Choline costs twice as much as the 30% MEA, yet it strips only 1/4 the square feet of photoresist, however it strips it very well. For the purposes of this talk, let us equate speed of stripping with quality of stripping, and of course, in general this is not a bad approximation. This kind of copper technology results in a speed versus number of panels stripped curve that looks like this:

Figure 4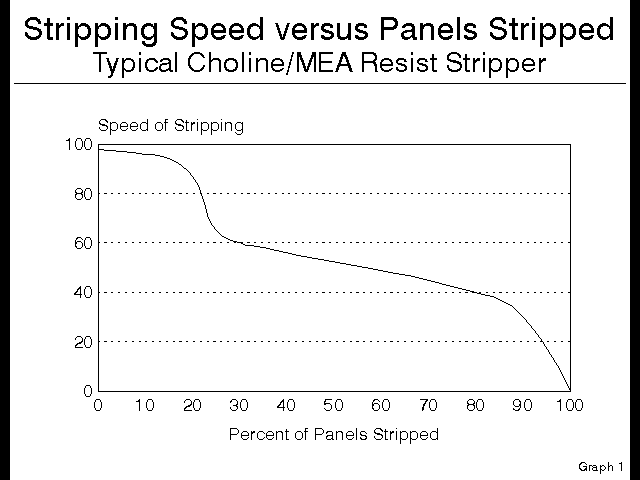
Notice that the speed over the first 20% of the stripper bath is very high, and almost completely uniform. Then as the Choline is depleted, the stripper starts stripping with the MEA, and consequently the speed drops suddenly and dramatically, and is uniform through it’s life. At this point the stripper is acting as if it were a pure MEA based product, and there was never any Choline present.
Luckily for the PCB fabricator, there is a gauge to tell exactly where a given bath is on this curve. This gauge is the pH of the bath. It is possible to replace the left axis of the speed versus panels graph above with a pH scale, and it will read just as true.
See this next graph:

Figure 5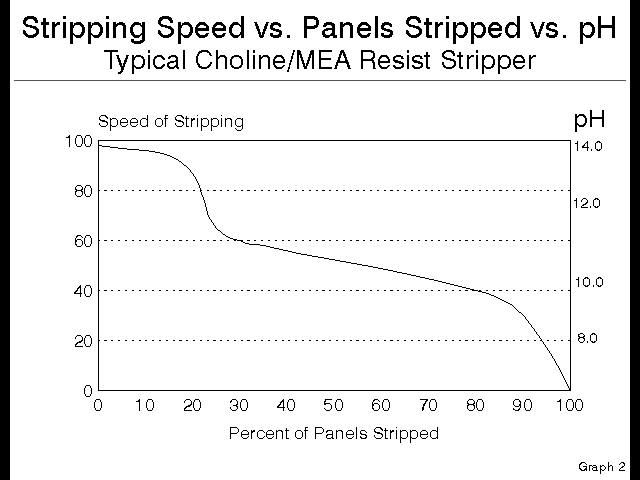
Notice that the Choline containing stripper starts at a pH over 13, and when the pH drops to 11.5 there is no Choline remaining, so all remaining stripping occurs with only the MEA. This is a handy way to control this stripping chemistry, and a much better approach than the traditional titration, which does not address quality or strength of amine present, but rather only looks at overall quantity of alkalinity present.
This is further why controlling this type of chemistry by monitoring the pH, not by titration, is the best way to insure the quality of stripping. The way that this is setup is to run the line until the minimum acceptable quality of stripping is observed, and then check the pH. This pH becomes the minimum acceptable at which the bath gives acceptable results.
But there is a larger issue to consider. This is the issue of whether or not it is appropriate to be using a stripping chemistry of this type at all, and whether or not it is not better to have two separate stripping chemistries, one for innerlayers, and a second for outerlayers.
As expensive as Choline is, it is better than producing rejects by trying to strip outerlayers using only MEA. However, if Choline is the critical key to stripping the outerlayer, then the intelligent PCB fabricator would dispose of this type of stripping chemistry after the Choline was consumed. This would result in effectively throwing away 80% of the stripping capacity of the product.
On the other hand, when stripping innerlayers, most PCB fabricators have no need for the expensive Choline in the product, since innerlayers are, relatively, so easy to strip. All of this assumes that the stripping chamber speed is not being pushed to it’s absolute maximum, in which case innerlayers can require Choline, and only Choline, as the key stripping ingredient. This implies that for most innerlayer stripping, an all MEA product would work very well, avoiding the high cost per square foot of resist stripped by Choline. Since the Choline is 65% of the cost of the popular stripping chemistries, by putting all that money into MEA instead of a Choline/MEA blend, it is possible to more than double the resist stripping capacity of the stripper without increasing the cost per gallon. This is the performance curve of an all MEA stripper.
As you can see from this graph:

Figure 6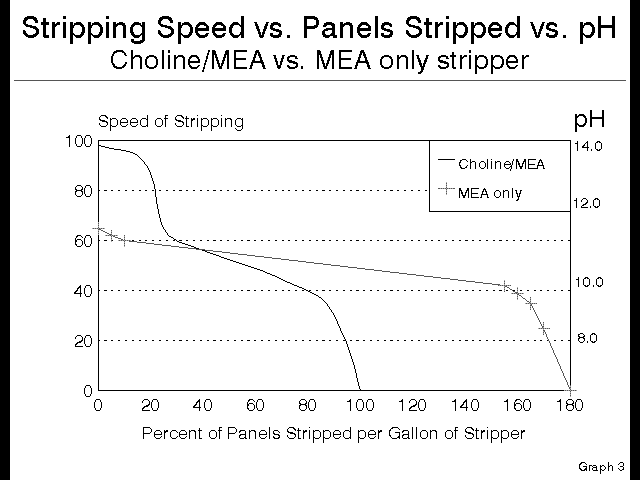
with an all MEA stripper, it is possible to save more than 50% of the stripping chemistry costs by using application-specific chemistry for each stripping installation. But let me make this even more clear with the next slide.
These are the chemical costs of the typical general purpose resist stripper.

Figure 7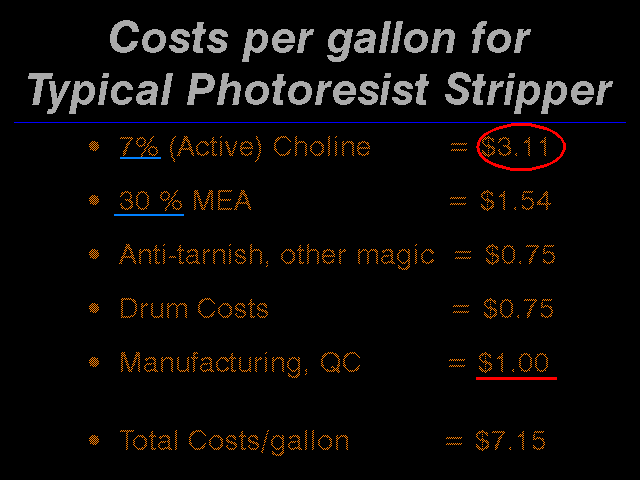
Looking at this it is obvious that we are in the wrong business, we should be selling Choline. But it is probably too late for that. As you can see, the Choline, which represents only 20% of the capacity of the stripper, is 2/3rds of the cost! It is clear that it is important to use Choline only when it is really required.
For outerlayers, a very high Choline product, with little or no MEA would give outstanding, consistent results, and although it would be very costly per gallon, it would be very inexpensive per square foot of resist stripped.
Innerlayers can be economically and completely stripped using a Choline-free stripper that costs no more per gallon than the conventional mixed amine strippers, but that gives a nearly 100% increase in square feet of resist stripped per gallon.
There are many other issues that can be discussed about resist stripping, such as the effect of solvents, and Choline replenishers. It is also easy for the PCB manufacturer to determine what chemistry is really required, and then specify that chemistry to it’s vendor. But this would require a longer time, and getting more specific, than is appropriate to this general talk.
I hope this has been enlightening, and that I spoke slowly enough for my competitors to take accurate notes. It would be a shame for them to foul up the details when they are developing strippers based on these concepts. More important, I hope that the PCB fabricators can now better understand a chemistry that is key to their manufacturing process, and understand that they have some options that will allow them to increase their yields, and lower their costs, both in original chemistry costs, as well as in waste treatment costs.